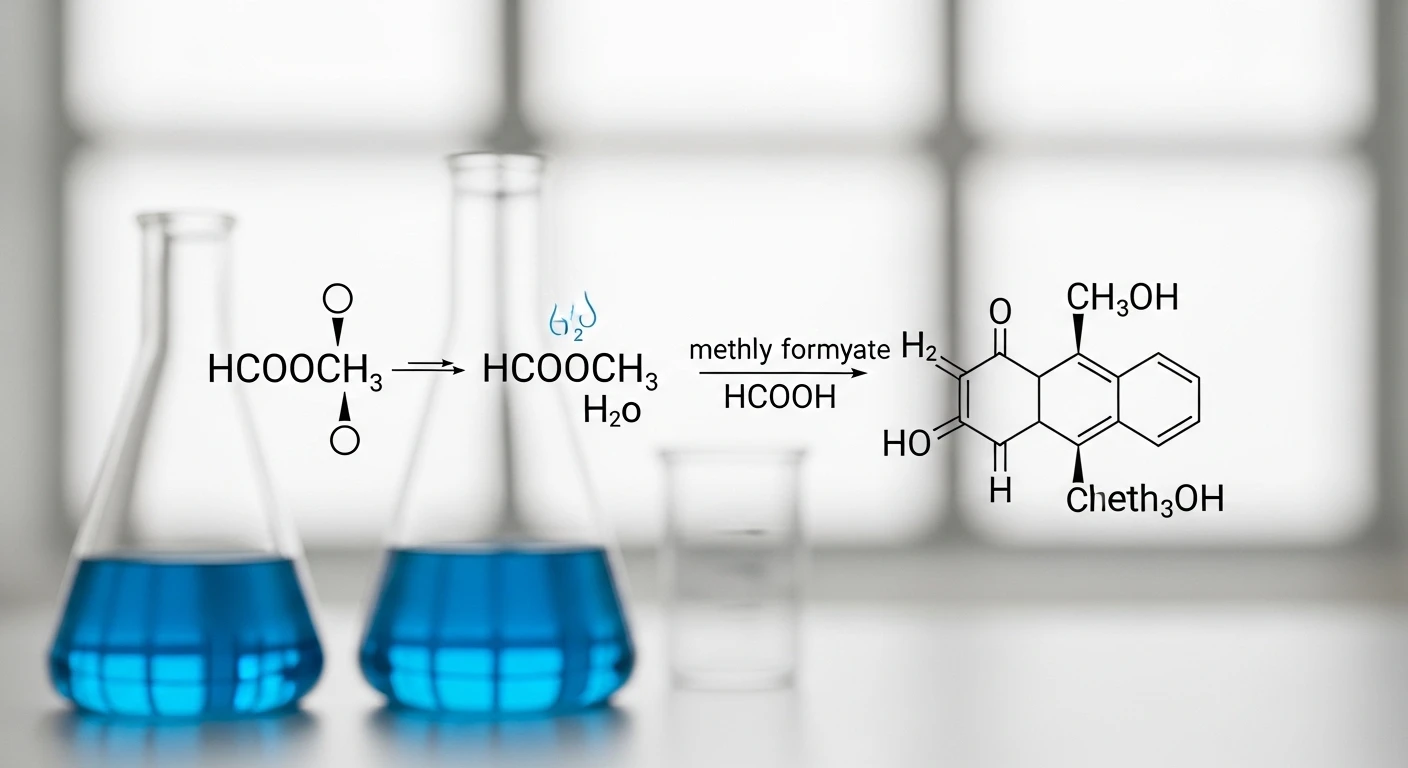
If you’ve stumbled upon the string hcooch ch2 h2o in a chemistry context, it might seem confusing at first glance. This arrangement of letters looks like a chemical formula but does not represent a single, isolated compound documented in scientific databases.
What it does represent is an important reaction theme in organic chemistry involving an ester a reactive carbon fragment and water. In this deep dive, you will learn what this notation really points to, how the underlying chemical processes work why water plays a central role and how these ideas show up in real laboratory and industrial contexts.
This article breaks down the meaning of the components, explains the mechanism at a practical level, and places the concept in broader chemical use.
What Is “HCOOCH CH2 H2O”?
At its core, hcooch ch2 h2o is not the name of a single molecule. Instead, it symbolizes an ester hydrolysis reaction system where methyl formate (an ester often written as HCOOCH₃) interacts with water, sometimes alongside reactive carbon fragments like methylene (CH₂). In organic chemistry, such combinations commonly illustrate how esters react with water to break into simpler products, revealing fundamental processes about how molecules transform under chemical conditions.
This notation, as seen in search results unites three elements:
- HCOOCH — typically a shorthand for methyl formate, an ester derived from formic acid.
- CH₂ — a methylene group that often appears in intermediate steps of organic reactions.
- H₂O — water, a ubiquitous solvent and reactant in hydrolysis and hydration reactions.
Taken together, these components often describe the interaction between organic esters and water a central theme in many chemical transformations.
Decoding the Chemical Components
Methyl Formate (HCOOCH₃) — The Ester
Methyl formate is the simplest ester of formic acid and methanol. Esters are a family of organic compounds known for their pleasant smells and roles as solvents in many industrial applications.
Key Characteristics:
- Molecular formula: C₂H₄O₂.
- Physical state: Volatile, colorless liquid.
- Uses: Solvent in coatings and resins, intermediate in chemical synthesis and precursor for formic acid production.
Methyl formate’s ester bond makes it reactive with water, especially under catalytic conditions. When water molecules attack this bond, the reaction can produce formic acid and methanol which we’ll explore next.
CH₂ — Methylene: A Reactive Fragment
The CH₂ group represents a methylene unit two hydrogen atoms attached to a carbon with two open valences (often depicted as a reactive intermediate in mechanisms).
In many textbooks and synthetic procedures, methylene units (CH₂) are involved in the building blocks of larger organic molecules, polymerization, or intermediate states in complex reaction sequences. They do not exist freely under normal conditions but play vital roles in organic synthesis where bonds are formed or rearranged.
When encountered in association with esters and water, CH₂ may signify reactive carbon fragments that participate in extended mechanisms beyond simple hydrolysis, such as chain growth or addition reactions.
H₂O — Water: The Universal Solvent
Water is both the medium and a reactant in many chemical reactions. Its polar nature enables it to dissolve a wide range of compounds and participate directly in chemical bond-breaking and bond-forming processes.
In the context of ester hydrolysis:
- Water attacks the ester bond, enabling the breakdown of methyl formate into formic acid and methanol.
- Water’s ability to donate and accept protons makes it critical in acid- and base-catalyzed mechanisms.
Water’s dual role as solvent and reactant helps drive the reaction forward, especially when catalysts are present.
The Central Reaction Mechanism: Ester Hydrolysis
The chemical scenario hinted at by “hcooch ch2 h2o” is most often seen in ester hydrolysis.
Balanced Reaction Equation
A clear way to represent the chemistry is:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
In words:
- Methyl formate + water yields
- Formic acid + methanol.
This equation is the foundation for understanding many processes in organic chemistry. It shows how an ester reacts with water and breaks the ester linkage, forming a carboxylic acid and an alcohol.

Acid-Catalyzed Hydrolysis
Under acidic conditions (like with sulfuric acid), the hydrolysis proceeds through these steps:
- Protonation of the carbonyl carbon increases its electrophilicity.
- A water molecule attacks the carbonyl carbon, forming a tetrahedral intermediate.
- Rearrangement breaks the ester bond, releasing methanol and forming formic acid.
This pathway is often used in laboratories and industry because it is efficient and predictable.
Base-Catalyzed Hydrolysis (Saponification)
Under basic conditions (using NaOH or KOH):
- Hydroxide ions attack the carbonyl carbon.
- The tetrahedral intermediate collapses, forming a formate salt and methanol.
- Acidification converts the formate salt into formic acid.
Both acid and base catalysis highlight how water participates as a reactant, and not just a passive solvent.
Practical Applications and Importance
Understanding this reaction system is more than an academic exercise. It has real industrial and technological relevance.
1. Industrial Production of Formic Acid
Formic acid, the product of the hydrolysis reaction, is used in:
- Textile dyeing and finishing.
- Leather processing.
- Feed preservatives in agriculture.
Formic acid also shows promise as a hydrogen carrier for fuel cell technologies, offering a potential pathway to cleaner energy.
2. Organic Synthesis and Chemical Manufacturing
Understanding the breakdown and formation of esters like methyl formate helps chemists design:
- Building blocks for pharmaceutical compounds.
- Intermediates in polymer and material production.
Water’s involvement as both reagent and medium aligns with principles of green chemistry, where safer and more sustainable processes are prioritized over hazardous organic solvents.
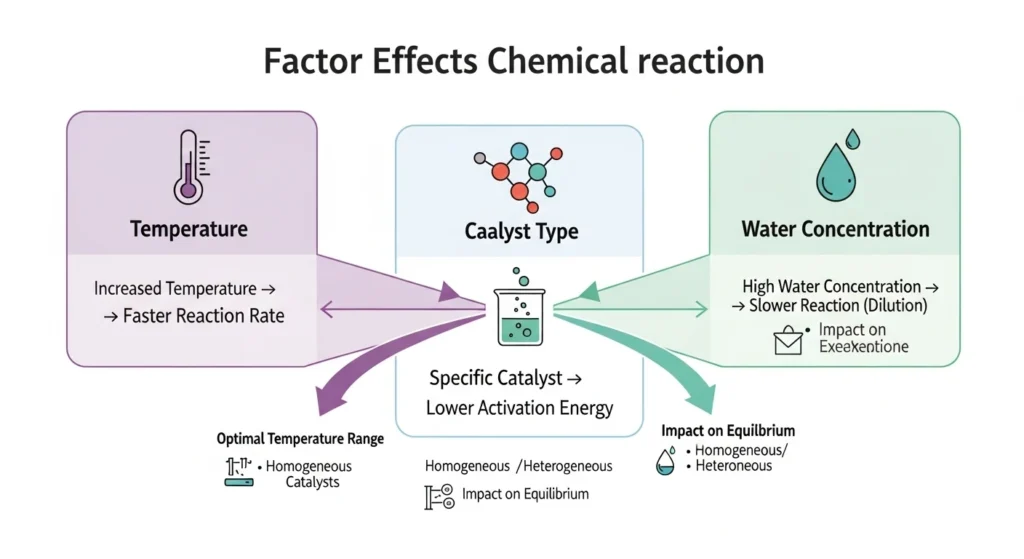
Factors Affecting the Hydrolysis Process
Several variables influence how efficiently this reaction proceeds:
- Temperature: Higher temperatures typically increase reaction rates but may also lead to side reactions.
- Catalysts: Acid or base catalysts accelerate hydrolysis.
- Water concentration: Excess water favors hydrolysis by shifting equilibrium.
These factors are crucial when scaling reactions for industrial processes.
Safety and Environmental Considerations
Working with reactive organic compounds requires careful attention to safety:
- Methyl formate is flammable and can cause respiratory irritation.
- Methanol is toxic and must be handled in well-ventilated areas.
- Formic acid is corrosive and should not contact skin or eyes.
- Waste streams from hydrolysis reactions must follow environmental regulations to prevent contamination.
Future Directions and Research Trends
Chemists continue to explore ways to make reactions involving these components more efficient and sustainable:
- Bio-based formic acid production from carbon dioxide is under investigation as a renewable pathway.
- Enzyme-catalyzed hydrolysis in water offers potential for milder, greener conditions.
- Flow chemistry systems integrate reaction and separation to improve yields and reduce waste.
These avenues show how simple molecular systems can remain at the forefront of chemical innovation.
Frequently Asked Questions
Is hcooch ch2 h2o a real molecule?
No. This string is a symbolic representation of a reaction system rather than a single compound.
What does this notation tell chemists?
It brings together an ester (methyl formate), a carbon fragment (CH₂), and water to describe ester hydrolysis and related reactions.
Why is water so important in this system?
Water acts both as a solvent and a reactant that enables bond cleavage and product formation.
What products come from this reaction?
The main products are formic acid and methanol.
Where is this chemistry used in the real world?
It appears in industrial production of chemicals, sustainable manufacturing, and educational labs.